Laboratory Tests
In the laboratory photoreactor of our own design, we can monitor simultaneously degradation of up to fifteen samples under controlled conditions.
We monitor the decrease of concentration of model compounds over time and simultaneously we determine the extent of their mineralization.
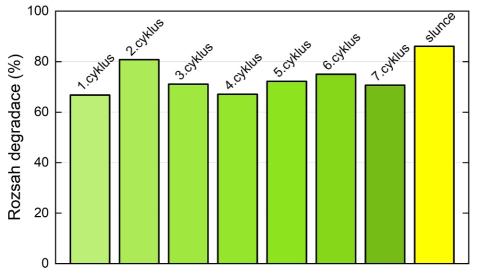
Testing through repeated cycles shows stable composite activity: the degradation of the herbicide 2,4-D dissolved in drinking water reaches 70% after 6 hours under an artificial light source and over 80% outdoors using natural sunlight (last column).
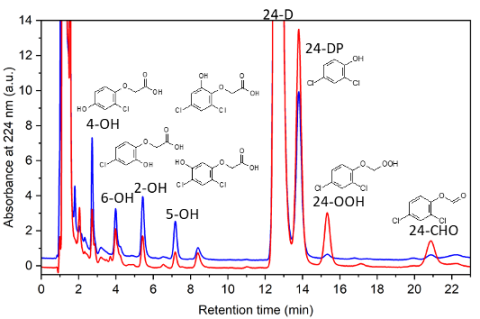
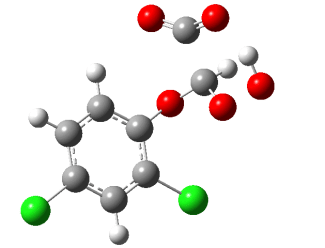
Our focus is not only on how quickly a specific pollutant can be removed from water. It is equally important to understand what is formed during its degradation — since intermediates may also be toxic or biologically active. Therefore, in addition to reaction rates, we concentrate on understanding degradation mechanisms.
We combine analytical methods such as liquid chromatography, which identifies emerging products, with kinetic studies and theoretical calculations that help unravel degradation pathways. This integrated approach ensures that our technology is safe, efficient, and free from undesirable by-products.